Expert and Research Work of the Nematology Group
- Diagnostics and consultancy: detection and identification of plant-parasitic nematodes (morphological, molecular-biological and biochemical species identification).
- Systematic surveys of quarantine species Globodera rostochiensis, G. pallida, Bursaphelenchus xylophilus.
- Control of other quarantine and economically important species (Ditylenchus dipsaci, D. destructor, Xiphinema americanum, X. index, Meloidogyne chitwoodi, M. fallax, Aphelenchoides besseyi, etc.).
- Morphological and genetic studies of potato cyst nematodes (species from genus Globodera), root-knot nematodes (genus Meloidogyne), virus-transmitting nematodes (genus Xiphinema, genus Longidorus), lesion nematodes (genus Pratylenchus).
- Studying the effect of plant-parasitic nematodes on plant physiology.
- Studying the biology of plant-parasitic nematodes (host plants status, developmental studies, nematode infestation modeling).
- Virulence and effectors of Potato Cyst Nematodes .
- Interactions: transmission of viruses with vectors of genus Xiphinema; interaction of nematodes of genus Meloidogyne and soil bacteria.
- Nematodes as biotic agents as part of IPM - Entomopathogenic nematodes.
- Plant-parasitic nematodes management.
Diagnostics and Consultancy
Plant-parasitic nematodes belong to the phylum Nematoda. They are the smallest and at the same time the most numerous eukaryotic animals on our planet. In the nematological laboratory, we perform identification of plant-parasitic nematodes species based on morphological, biochemical and genetic characteristics.
Morphological analyses of nematodes are performed with modern light microscopes by analysing species-specific morphometric parameters. Based on measured and visually determined parameters, we determine the species of the nematodes.
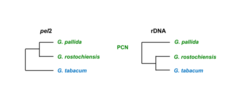
Due to the large number of species and morphological similarity between them, it is recommended to determine the nematode species with an additional independent method. For this purpose, we use biochemical (we analyse isoenzymes and total proteins using electrophoresis) and genetic approaches (most often we analyse ITS region of ribosomal rDNA by various methods such as: PCR, PCR-RFLP, PCR in real-time and by determining nucleotide sequence).
The Isoenzyme Gel Electrophoresis Method
The malate dehydrogenase (MDH) and esterase (EST) isoenzymes are species specific in root-knot nematodes. For identification, we use freshly isolated females from infested plant tissue, isolate the total proteins and separate them using protein electrophoresis. After the electrophoresis, we detect the MDH and EST spots with enzyme reaction and determine their migration coefficients. M. javanica electrophoretic sample is used for reference.
Besides species identification, we advise our clients about the suitable ways of controlling and management of the of detected nematode species.
Special Control of Quarantine Nematodes
We are part of special controls of quarantine nematodes.
Pine wood nematode (Bursaphelenchus xylophilus) is classified as an extremely dangerous pest in forestry; it primarily endangers pine wood (genus Pinus) and to a smaller extent other coniferous trees such as larch, fir and spruce trees (Larix, Abies, Picea). Bursaphelenchus xylophilus has not been detected in Slovenia yet. By systematically monitoring the pine wood nematode, we wish to prevent its introduction and spread across the country. The work is performed in accordance with the Rules on the Measures to Prevent the Introduction and Spread of Pinewood Nematode (Official Gazette of the RS, no. 45/09).
Potato cyst nematodes (Globodera rostochiensis and G. pallida) are dangerous parasites of the Solanaceae plants and cause significant decrease potato yield. In Slovenia, we have confirmed the presence of yellow potato cyst nematode G. rostochiensis and white potato cyst nematode G. pallida. With a systematic monitoring, we prevent new introductions and spread of potato cyst nematodes as dictated by the Rules on Measures for the Prevention of Spread and Suppression of Potato Cyst Nematodes (Official Gazette of the RS, no. 49/10 of 18 June 2010).
Nematodes that Transmit Plant Viruses
Virus-transmitting nematodes on crops cause indirect and direct economic damage. Direct damage is smaller and occurs due to the nematode feeding on the host’s roots, while the indirect damage can be quite large. Nepoviruses are primarily parasites of indigenous plants and cause significant economic damage mainly on fruit trees and grapevines and can even cause the decay of entire fruit trees and vines. To a smaller extent, nepoviruses can spread physically, through direct contact of plants and via seeds and pollen. The most important way of spreading is through their vectors, nematodes from the Xiphinema genus, which are present in the soil, even at greater depths (2 - 3 metres), and are highly polyphagous, which practically prevents their eradication.
In our research of the Xiphinema rivesi species that is present in Vipava Valley, we revealed that it can transmit tobacco ringspot viruses (TRSV) and tomato ringspot viruses (ToRSV). Thus, for the first time, we proved the transmission of quarantine nepoviruses TRSV and ToRSV with nematodes that are present in Europe.
We are also focused on researching the interaction between the Xiphinema index nematodes and the grapevine fanleaf virus (GFLV), which causes increasingly greater damage to the producers of grapevines in the Primorska region.
Control and Containment of Plant-Parasitic Root-Knot Nematodes (Meloidogyne spp.)
Control of root-knot nematodes (Meloidogyne spp.) is extremely difficult. They can parasitise numerous plant species; besides many herbaceous species, they also infest a great number of woody plants. Consequently, crop rotation in the sense of their containment is largely ineffective. In Slovenia the available chemical plant protection products (nematocides) are limited and the management of these pests is therefore difficult. With the purpose of developing new effective and environmentally-friendly approaches for controlling the root-knot nematodes, we are focusing our research towards developing of alternative approaches for controlling plant-parasitic nematodes, such as using biofumigation (controlling soil pests by using volatile chemical substances, e.g. glucosinolates, during the decomposition of ploughed above-ground and below-ground parts of selected species of crucifers), the use of various plant extracts and searching for the resistance sources in crops and wild relatives from the Agricultural Institute of Slovenia Gene Bank.
.
Screenings for sources of resistance in lettuce to Meloidogyne spp.
Article: Biological Control of Root-Knot Nematodes (Meloidogyne spp.): Microbes against the Pests
Prenos datotek
- Borova ogorčica PDF 530 kb
- Krompirjeve ogorčice PDF 680 kb
- Ogorčice koreninskih šišk PDF 433 kb
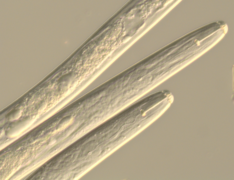 Prednji del ličink Globodera spp.
Prednji del ličink Globodera spp.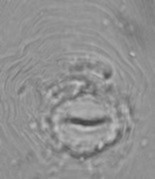 Spolno-analni del samice Meloidogyne spp.
Spolno-analni del samice Meloidogyne spp.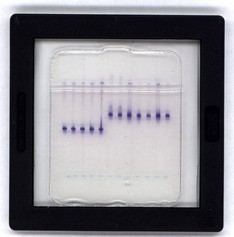 Elektroforegram izoencima esteraza za vrsto Meloidogyne sp.
Elektroforegram izoencima esteraza za vrsto Meloidogyne sp.